- Main Menu
- What is a Pap Smear and Why is it Performed?
- Who Should Receive a Pap Smear?
- What are the Latest Statistics on Pap Smears and Cervical Cancer?
- What is the Human Papillomavirus (HPV)?
- How Should a Woman Prepare for a Pap Smear?
- How is a Pap Smear Performed?
- Are There Any Risks to a Pap Smear?
- How are Cervical Cells Evaluated?
- How are the Results of a Pap Smear Described?
- What are the Latest Advances with Pap Smear?
- What Can Affect the Results of a Pap Smear?
- What Additional Tests May Be Ordered if the Results of a Pap Smear are Abnormal?
- Additional Resources and References
- What is a Pap Smear and Why is it Performed?
- Who Should Receive a Pap Smear?
- What are the Latest Statistics on Pap Smears and Cervical Cancer?
- What is the Human Papillomavirus (HPV)?
- How Should a Woman Prepare for a Pap Smear?
- How is a Pap Smear Performed?
- Are There Any Risks to a Pap Smear?
- How are Cervical Cells Evaluated?
- How are the Results of a Pap Smear Described?
- What are the Latest Advances with Pap Smear?
- What Can Affect the Results of a Pap Smear?
- What Additional Tests May Be Ordered if the Results of a Pap Smear are Abnormal?
- HPV Test Effective for Women with Abnormal Pap Smears
- Additional Resources and References
Cervical cancer screening began in the United States in the late 1940s after Dr. George Papaniclaou developed the Pap smear. A Pap smear (also called a Pap test) is a screening test used to examine cells from the cervix and the vagina. (The cervix is the portion of the uterus that protrudes into the vagina). Cervical and vaginal cells are studied to determine whether there is evidence of cancer or pre-cancerous changes. If abnormal cells are found, they are classified according to their degree of abnormality. Most abnormal Pap smears are caused by cervical infections or inflammation which can usually be successfully treated before leading to cancer.
Abnormal Pap smear findings may indicate:
- Infection (including the human papillomavirus, HPV)
- Swelling or inflammation
- Pre-cancerous cell changes
- Cervical cancer
Pap smear is perceived by the medical community as a reliable screening tool to help detect cervical cancer, along with regular pelvic exams. However, Pap smears cannot typically detect other types of cancers, such as ovarian or uterine cancers, or sexually-transmitted diseases (STD), with the exception of the human papillomavirus (HPV).
The American Cancer Society recommends that all women begin receiving yearly Pap smears and pelvic examinations at age 18 or when they become sexually active, whichever occurs earlier. Some physicians will not perform a Pap smear each year if a woman has had three negative Pap smears in the course of three years. However, a yearly pelvic exam should be continued even if Pap smears are not given each year.
Regular or more frequent Pap smears may be performed on women who have had hysterectomies (surgery to remove the uterus, including the cervix), especially if the hysterectomy was performed because pre-cancerous and cancerous cells were found in the cervix. In women who have had hysterectomies, the tissues of the vagina are analyzed for changes that may indicate cancer.
In addition, women who have a weakened immune system (from AIDS, chemotherapy or drug treatments, or organ transplants) are considered at higher risk for cervical cancer and may require Pap smears more frequently than once a year.
Approximately 50 million Pap smears are performed each year in the United States. The death rate from cervical cancer has declined dramatically since 1955 (74% from 1955 to 1992) largely from the increased use of the Pap smear. When detected early, the five-year survival rate for cervical cancer is approximately 91%. If cervical cancer is detected before it has invaded any surrounding tissues, the five-year survival rate is nearly 100%.
Even with the increased use of the Pap smear, between 60% and 80% of American women who are newly diagnosed with cervical cancer have not had a Pap smear within the last five years, if ever. According to recent studies, elderly, African-American, and low-income women are the least likely to have annual Pap smears.
The American Cancer Society estimates that in 2001, 12,900 new cases of invasive cervical cancer will be diagnosed and approximately 4,400 women will die from the disease. Researchers estimate that non-invasive cervical cancer is nearly four times as likely as invasive cervical cancer.
The human papillomavirus (HPV) is a common sexually transmitted disease that affects both men and women. There are over 80 different strains of HPV, and the most do not pose any health risks. However, some of the strains of HPV cause genital warts in men and women and can cause cellular changes that may lead to cervical cancer in women. A major risk factor for cervical cancer is the HPV. It is estimated that one million new cases of HPV occur each year, and 20% to 40% of sexually active women have HPV.
It is essential that all women over age 18 have annual Pap smears since the test accurately detects approximately 90% of all cervical cancers. HPV is most commonly discovered by abnormal Pap smears results and is most likely to spread when genital warts are visible. HPV does not typically interfere with pregnancy or childbirth.
Research has shown that condoms do not always prevent the spread of HPV because the virus can be transmitted by skin contact of any HPV-infected area (such as the genitals). Limiting the number of sexual partners and not having sexual intercourse with a person who has had several sexual partners can help prevent HPV. There is currently no cure for HPV.
According to the American Cancer Society, removing genital warts and abnormal cell growths caused by HPV can help reduce the risk of cervical cancer from HPV. Treatments to remove genital warts include: laser surgery, convention surgery, cold cautery (freezing the tissue), hot cautery (burning the warts off with an electric instrument), or directly applying podophyllin or trichloroacetic acid. In addition, the U.S. Food and Drug Administration (FDA) has recently approved a drug called imiquimod cream to treat genital and perianal warts.
Click here to learn about HPV testing.
Women should not schedule a Pap smear while they are menstruating because the presence of blood cells may interfere with the test results. However, if a woman is experiencing abnormal vaginal bleeding, a Pap smear may help determine the cause. Many physicians recommend not using douches, tampons, or vaginal medications for at least 24 to 48 hours prior to having a Pap smear. According to the American Cancer Society, the ideal time for a woman to have a Pap smear is five days after her menstrual period has ended.
Patients will be asked to remove clothing below the waist and drape a paper cloth around the waist prior to the Pap smear. A nurse or other healthcare professional maybe present to assist the physician. To perform a Pap smear, the physician will begin by inserting a metal or plastic instrument (called a speculum) into the vagina to keep it open so that the cervix may be clearly seen. Next, he or she will use a small brush, cotton-tipped swab, or wooden spatula to obtain a sample of cells and mucus from the outer part of the cervix (the ectocervix). For women who have had their uteruses removed, a sample of vaginal cells is collected. The samples of cells and fluid are then smeared on glass slides and taken to the lab for examination under a microscope.
After the Pap smear is completed, the physician will usually perform a pelvic exam to check the woman’s uterus, vagina, ovaries, and fallopian tubes for any abnormalities in shape or size. Typically, the Pap smear and pelvic exam take only a few minutes to complete. Though most women do feel some discomfort, pressure, or cramping during the exams, neither test should be painful. Women with tender, narrow, or irritated vaginas may experience more discomfort than others. Some women experience slight vaginal bleeding after the Pap smear is completed.
Pap smears and pelvic exams may be performed by physicians, physician assistants, nurse practitioners, or other specially trained medical professionals.
While there is a very remote chance of infection from a Pap smear, there are generally no risks.
After the physician obtains the cervical cells, he or she will usually give the specimen to a nurse, physician’s assistant, or other specially trained medical professional in the exam room who will smear them on glass slides and take them to the lab to be evaluated. A new way of collecting cervical cells involves placing the sampling device (spatula, brush, or broom) directly into a liquid (see section below). The cells are then placed on slides in the laboratory.
In the lab, a pathologist or specially trained laboratory technologist will analyze the cells under a microscope to determine whether they are cancerous, pre-cancerous, or benign (non-cancerous).
Pap smear results are usually available to patients within two weeks. The Bethesda System (TBS) is the most commonly used system to describe Pap smear results, though some labs use older system such as the CIN (cervical intraepithelial neoplasia) system or the Class system. The Bethesda system involves using a number of descriptive terms instead of a number system.
First, the technologist or physician will determine whether the cell sample is satisfactory for evaluation. If it is, he or she will proceed with the analysis. A common reason why the cells may not be satisfactory for evaluation is that too few cells were removed during the Pap smear. In this case, the Pap smear should be repeated.
If no abnormalities are found, the Pap smear results are called negative. If an abnormality is found, then the results of the test are positive.
The following terms may be used to describe abnormal cervical cells:
- ASCUS: Minor cells changes of unknown cause. The situation will be assessed.
- LSIL: Minor cell changes unlikely to progress to cancer. For example, Certain strains of the human papillomavirus (HPV) may cause genital warts.
- HSIL: Cell changes that may progress to or are in the early stages of cancer.
- SIL: Abnormal cells are present in the cervix. The term CIN is accompanied by a number (1 to 3) to describe how much of the cervix contains abnormal cells.
- CIN 1: Mild cellular dysplasia. The term dysplasia is used to describe cells that undergo a series of changes in their appearance. They appear abnormal under a microscope but do not invade nearby healthy tissue.
- CIN II: Moderate cellular dysplasia.
- CIN III: Severe cellular dysplasia.
- CIS: Carcinoma in situ. The term "in situ" literally means "in place." Cancer is present but has not spread into nearby tissue.
- Cervical cancer
- Endometriosis: Cells from the lining of the uterus (endometrial cells) have moved outside of the uterus to the cervix.
- AGUS: Glandular cells are found and the reason for their presence on the cervix is unknown.
- Glandular cancer: Cancer from glandular cells in the cervix.
- Endometrial cancer: Cancer from cells that line the uterus (endometrial cells).
- Other types of cancer: Cancer that has spread to the cervix from other parts of the body.
- Other types of glandular cell cancer:Glandular cell cancer that has spread to the cervix from other parts of the body.
If a patient has had her uterus removed (hysterectomy), then cells from the vagina will be tested for abnormalities. In this case, the description of the laboratory analysis is typically as follows:
- Normal findings for the patient’s age and medical history.
- Abnormal findings for the patient’s age and medical history, followed by specific reason.
- Evaluation not possible, followed by specific reason.
Recently, new techniques have been developed to further improve cervical cancer cell sample collection and specimen quality. Hologic’s ThinPrep System and MediSpectra, Inc.’s LUMA Cervical Imaging System are two such new techniques that have been approved by the U.S. Food and Drug Administration (FDA). While the conventional Pap smear is still an accurate method of detecting abnormalities or cancer, research has shown that these new techniques may be more effective at detecting cervical cancer and pre-cancerous conditions than the conventional Pap smear by:
- making the patient’s slide more representative of the patient’s clinical condition
- improving the preservation of the sample
- standardizing the presentation of cells on the slide
- reducing mucus, blood, or other debris that may eclipse pre-cancerous or cancerous cells
A 1991 study of 600 laboratories found that up to 20% of Pap smear slides are unsatisfactory for evaluation and 40% are satisfactory but of limited value. Reasons for these classifications include too few cells to evaluate or too much mucus or blood in the sample to make an accurate interpretation.
With the conventional Pap smear technique, cervical cells are collected with a small stick or spatula and smeared on a slide for pathological analysis. However, a 1994 study published in the American Journal of Clinical Pathology found that up to 80% of a sample taken from a patient using the conventional Pap smear technique is not smeared on the slide but remains on the collection device. Instead of smearing the cervical cells on a slide after they are removed from the patient, new "direct-to-vial" techniques involve immediately rinsing the collected cells in a vial filled with a special solution. This reduces the likelihood that a patient’s cell sample will be damaged by air, clumping, etc.
The vial is then taken to the laboratory for slide preparation and screening. In the laboratory, the vial is inserted in a sample preparation device which breaks up blood, mucus, and other problematic materials. The thin layer of cells in then transferred to a slide and is automatically deposited into a preservative solution. With these newer methods, physicians are also able to conduct multiple analyses (such as HPV testing) using residual cells collected in the vial instead of having to order an additional Pap smear.
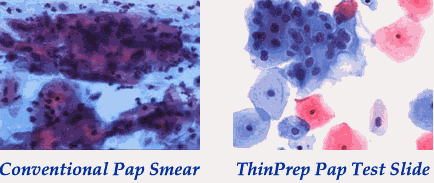 |
With conventional Pap smear, cells can be obscured by blood, mucus or clumping. With direct-to-vial techniques such as the ThinPrep method, more cells are preserved and there is less overlapping, blood, mucus, etc. Images courtesy of Hologic/Cytyc. |
In a clinical trial of 6,747 patients conducted by Cytyc, the maker of the ThinPrep direct-to-vial technique, researchers found a 65% improvement in the detection of cervical cancer at three screening centers using this new technique and a 6% improvement at three hospitals where the incidence of cervical cancer is historically high. The direct-to-vial technique was also found to be more effective at detecting severe cervical lesions than the conventional Pap smear. In all, more than 30 major studies including more than 300,000 patients in the United States, Europe, Asia, Africa, and Australia have found that "direct to vial" Pap smear techniques have benefits over the conventional cervical cell collection.
While new direct-to-vial methods have been shown to be more effective at detecting cervical cancer than the conventional Pap smear, the cost of these new methods is higher. Some insurance companies do provide coverage for these newer techniques while others do not. Therefore, women should check with their insurance companies prior to choosing these newer techniques. In some cases, women who choose to have the new direct-to-vial sample collection will have to pay out-of-pocket for the additional cost of the test.
Another advance in Pap smear screening is the use of computerized instruments that can recognize abnormal cells in Pap smears (similar to the use of computer-aided detection with mammography). An example of this technology is the AutoPap system made by Tripath Imaging, Inc. Normally, technologists and physicians evaluate all Pap smear samples. However, with this technology, a computer re-examines the sample and marks areas of the sample that may indicate abnormal cells. The technologist or physician then takes a closer look at these areas. The advantage of this technology is that the computer instruments may find pre-cancerous or cancerous cells that a technologist or physician may miss. However, some physicians believe that the technology can lead to a significant number of "false positive" results (the technology falsely indicates that the sample contains abnormal cells). These false positive results can lead to unnecessary repeat Pap smears, colposcopy, or other exams. As with the "direct to vial" techniques, this method may or may not be covered by insurance. Nevertheless, with continued improvements, many physicians believe this type of technology will eventually lead to more accurate detection of cervical cancer and pre-cancerous conditions.
Menstrual blood, vaginal lubricants, douches, or vaginal medications may cause inaccurate results of a Pap smear. Also, failure to apply a preservative to the slide sample immediately after cervical cells are obtained and spread onto the slide may cause the cells to become dried out.
- Colposcopy: The cervix is viewed through a colposcope (an instrument with magnifying lenses) to check for abnormalities.
- Cervical biopsy: A portion of tissue from the cervix may be removed for further examination and to confirm if cancer is present.
- Cone biopsy: An elaborate cervical biopsy, a cone biopsy involves removing a cone-shaped region of tissue high on the cervical (that would not be seen with a colposcopy).
Click here to learn more about how cervical cancer is diagnosed.
- The National Cancer Institute provides information on Pap smears at http://cancernet.nci.nih.gov/
- The American Cancer Society provides information on Pap smears at http://www3.cancer.org/cancerinfo/load_cont.asp?ct=8&doc=26
Updated: November 21, 2007



